Direct Observation of Processive Movement by Individual Myosin V Molecules
Takeshi Sakamoto1, Ichiro Amitani1, Etsuo Yokota2, and Toshio Ando1#
1
Department of Physics, Faculty of Science, Kanazawa University, Kanazawa 920-1192, Japan, 2Department of Life Science, Faculty of Science, Himeji Institute of Technology, Himeji 678-1297, Japan@
ABSTRACT@(Biochem. Biophys. Res. Commun. 272:586-590 (2000))
The full text
PDF file is available.Myosin V is an unconventional myosin thought to move processively along actin filaments. To have hard evidence for the high processivity, we sought to observe directly the movement by individual native chick brain myosin V (BMV) molecules with fluorescent calmodulin. Single BMV molecules did exhibit highly processive movement along actin filaments fixed to a coverslip. BMV continued to move up to the barbed end of its actin track, and did not readily detach from actin. The barbed end, therefore, got brighter with time, because of a constant stream of BMV traffic. The maximum speed of the processive movement was 1Êm/sec, and the maximum actin-activated ATPase rate was 2.4 s-1. These values apparently imply that
BMV travels a great distance, 400nm, per an ATPase cycle.

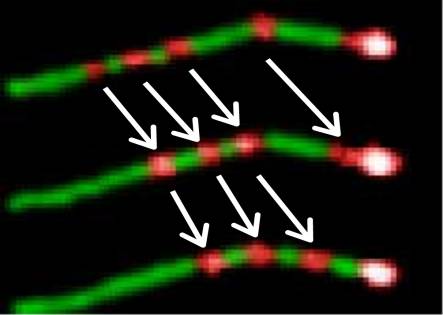
Processive movement by individual BMV molecules along an actin filament. Successive video images at 1s intervals showing processive movement by single molecules of BMV along a fluorescent actin filament. The actin filament is labeled with RGP. The four light spots are moving toward the stationary light spot at the actin-filament end. KCl: 75 mM. The barbed end of the actin filament is brighter because of a constant stream of BMV traffic.